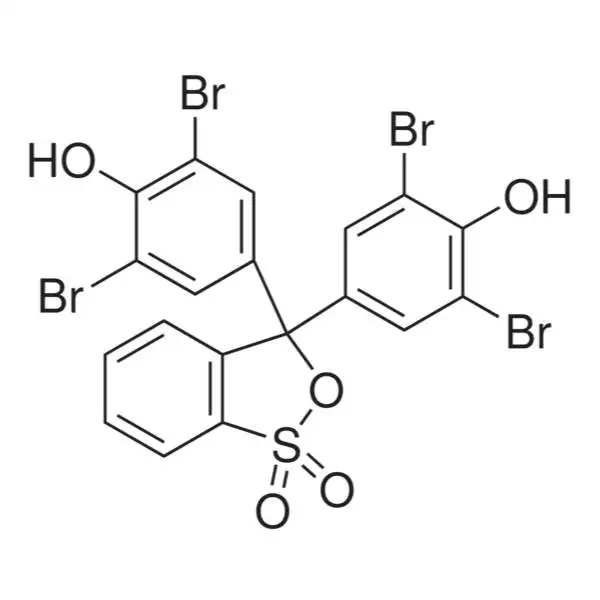
Bromophenol Blue
Bromophenol Blue is an acid-base indicator since its useful range lies between pH 3.0 and 4.6. It changes from yellow at pH 3.0 to blue at pH 4.6; this reaction is reversible. Bromophenol Blue is structurally related to phenolphthalein (a popular indicator). BPB is also used as a color marker to monitor the process of agarose gel electrophoresis and polyacrylamide gel electrophoresis They are used in inks, paints, and as indicators and reagents. Concentrations of BPB in plasma, urine, and bile were determined spectrophotometrically after intravenous bolus injections and infusions in rats. BPB is the most widely used stain in gel electrophoresis for tracing the migration of samples on electrophoretic gels.
Royal Industries’ Basic Yellow 1 Ensures High-Purity and Strong Affinity in Every Application.