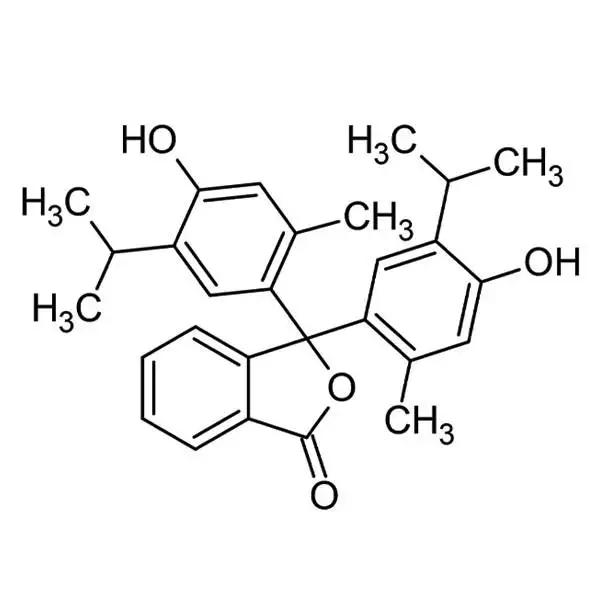
Thymolphthalein
CAS Number: 125-20-2
Molecular Weight: 430.54
MDL number: MFCD00005909
EC Index Number: 204-729-7
Analysis Note
- Identity (UV/VIS-Spectrum): passes test
- Appearance: White to almost white powder, eventually slightly yellowish.
- Clarity of solution (1 g/l; ethanol): passes test
- Absorption maximum λmax. (buffer pH 10.5): 592 – 596 nm
- Spec. Absorptivity A 1%/1cm (λmax; 0.01 g/l; buffer pH 10.5; calculated on anhydrous substance): 800 – 900
- Transition range: pH 9.0 – pH 10.5 colourless – blue
- Transition range (according to ACS): passes test
- Transition interval (according Reag. Ph. Eur.): passes test
- Loss on drying (110 °C): ≤ 1 %
Royal Industries’ Bromothymol Blue for Reliable pH Monitoring in Research and Industry.
| grade | ACS reagent |
| Quality Level | 200 |
| Agency | reag. Ph. Eur. |
| form | solid |
| loss | ≤1% loss on drying, 110°C |
| color | white to almost white |
| visual transition interval | 9.0-10.5, colorless to blue |
| mp | 253 °C |
| bulk density | 400 kg/m3 |
| λmax | 592-596 nm (buffer pH 10.5) |
| InChI | 1S/C28H30O4/c1-15(2)20-13-23(17(5)11-25(20)29)28(22-10-8-7-9-19(22)27(31)32-28)24-14-21(16(3)4)26(30)12-18(24)6/h7-16,29-30H,1-6H3 |
| InChI key | LDKDGDIWEUUXSH-UHFFFAOYSA-N |